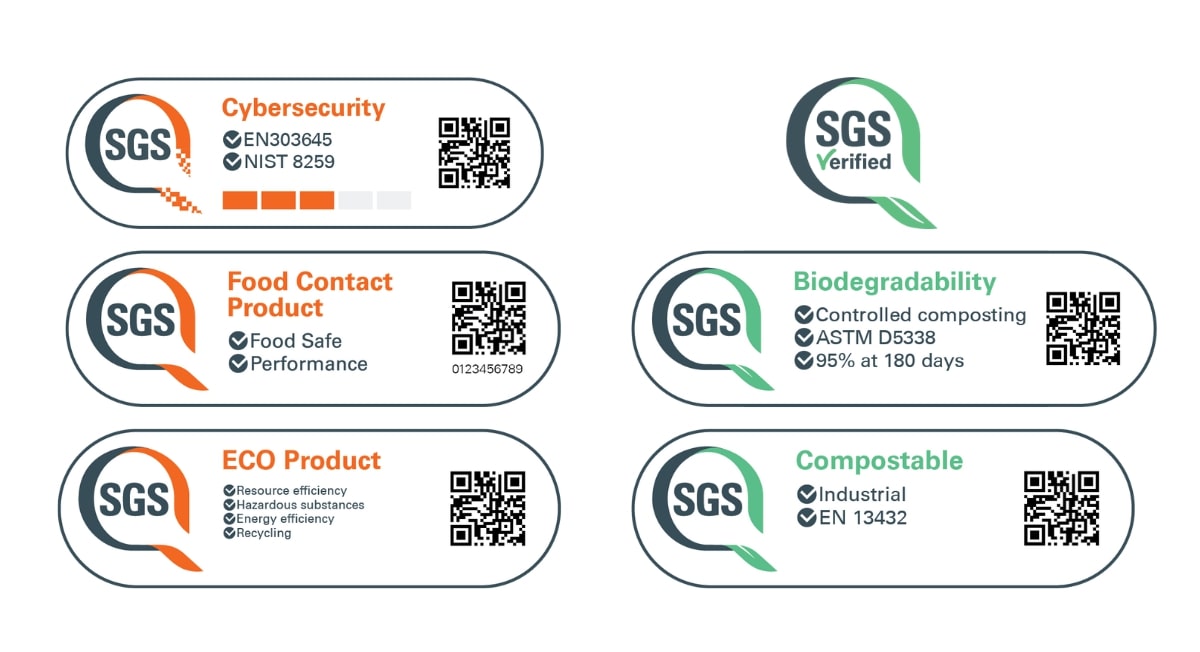
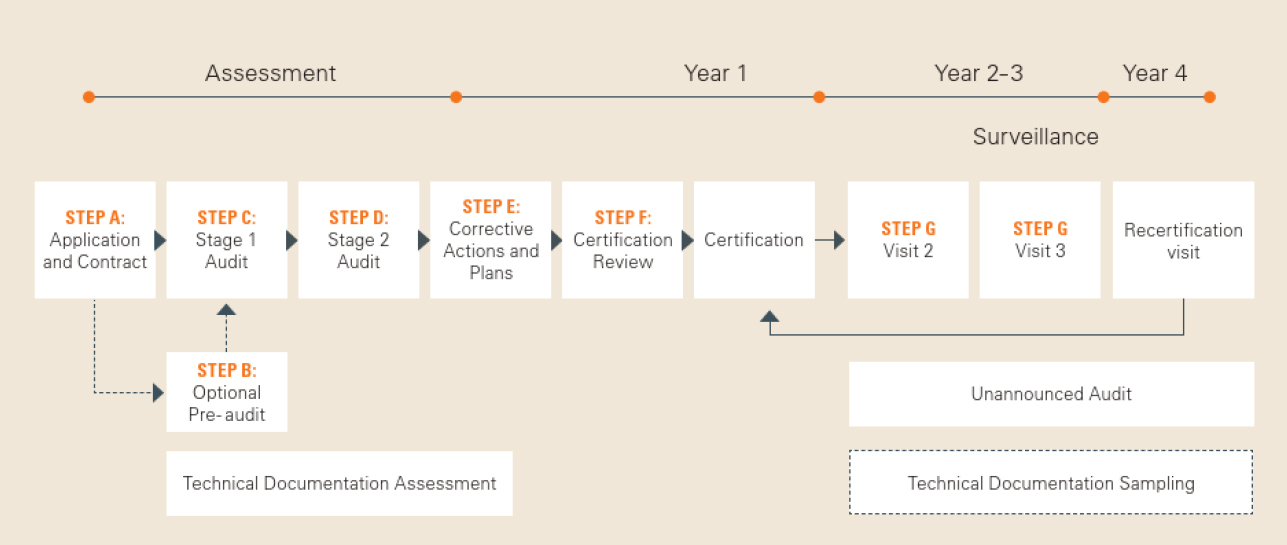
Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

Organismos Notificados MDR (25): SGS Belgium (Belgica) ON num. 1639 nuevo ON. Enhorabuena @SGS_Spain !!!

Ilkka Juuso on LinkedIn: It's time for a GRAND NEW ADVENTURE! Today I dive several fathoms deeper… | 82 comments

CG500B Disposable Hooded coverall has recently received CE Certification from the CE Notified Body, SGS | Shanghai C&G

CG500B Disposable Hooded coverall has recently received CE Certification from the CE Notified Body, SGS | Shanghai C&G

SITEC - 93/42/EEC – MEDICAL DEVICES DIRECTIVE, CE MARKING FOR EUROPE SITEC Private Limited is Certified by SGS for Directive 93/42/EEC for Class IIB and Class III Medical devices. The Certificate represents