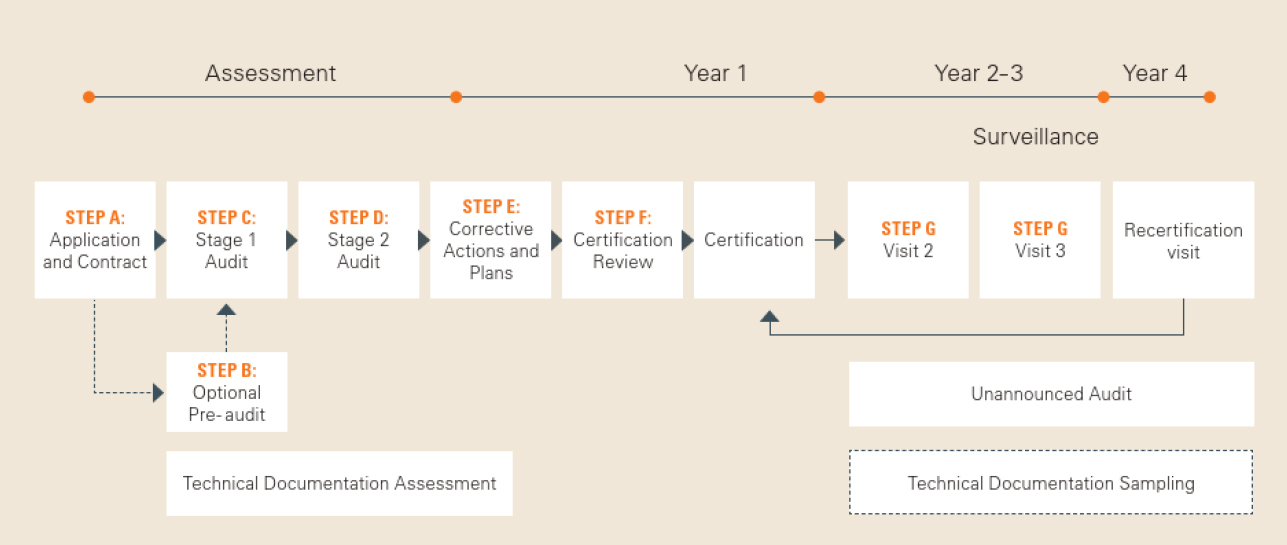
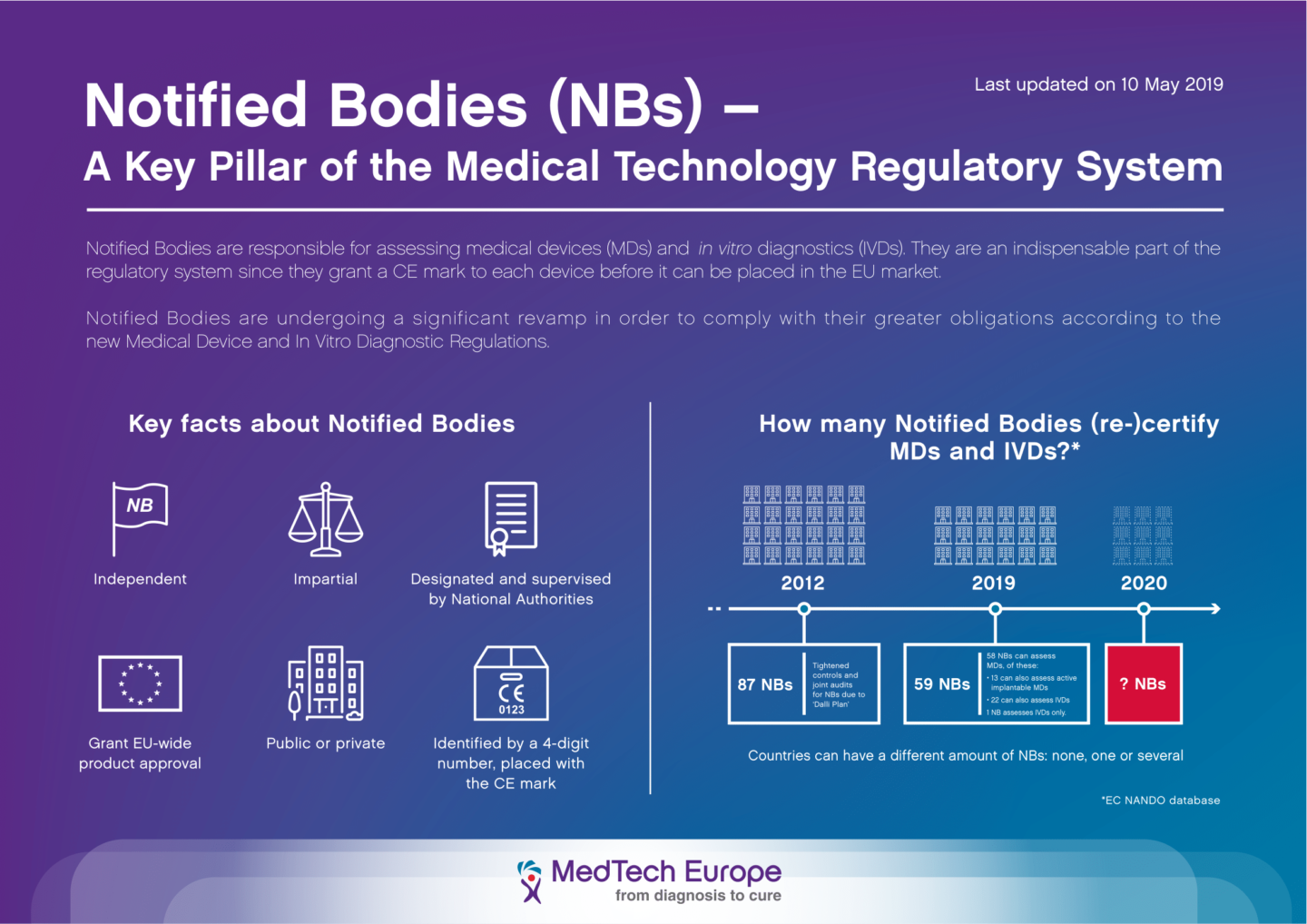
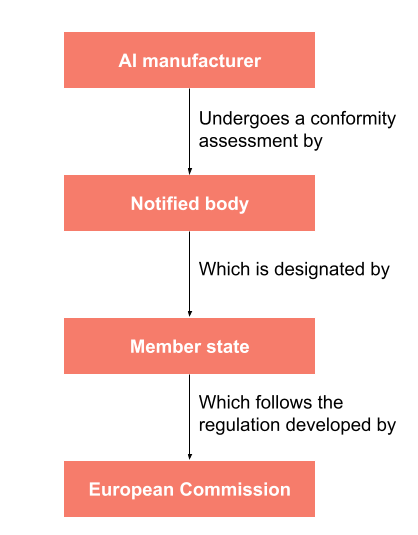
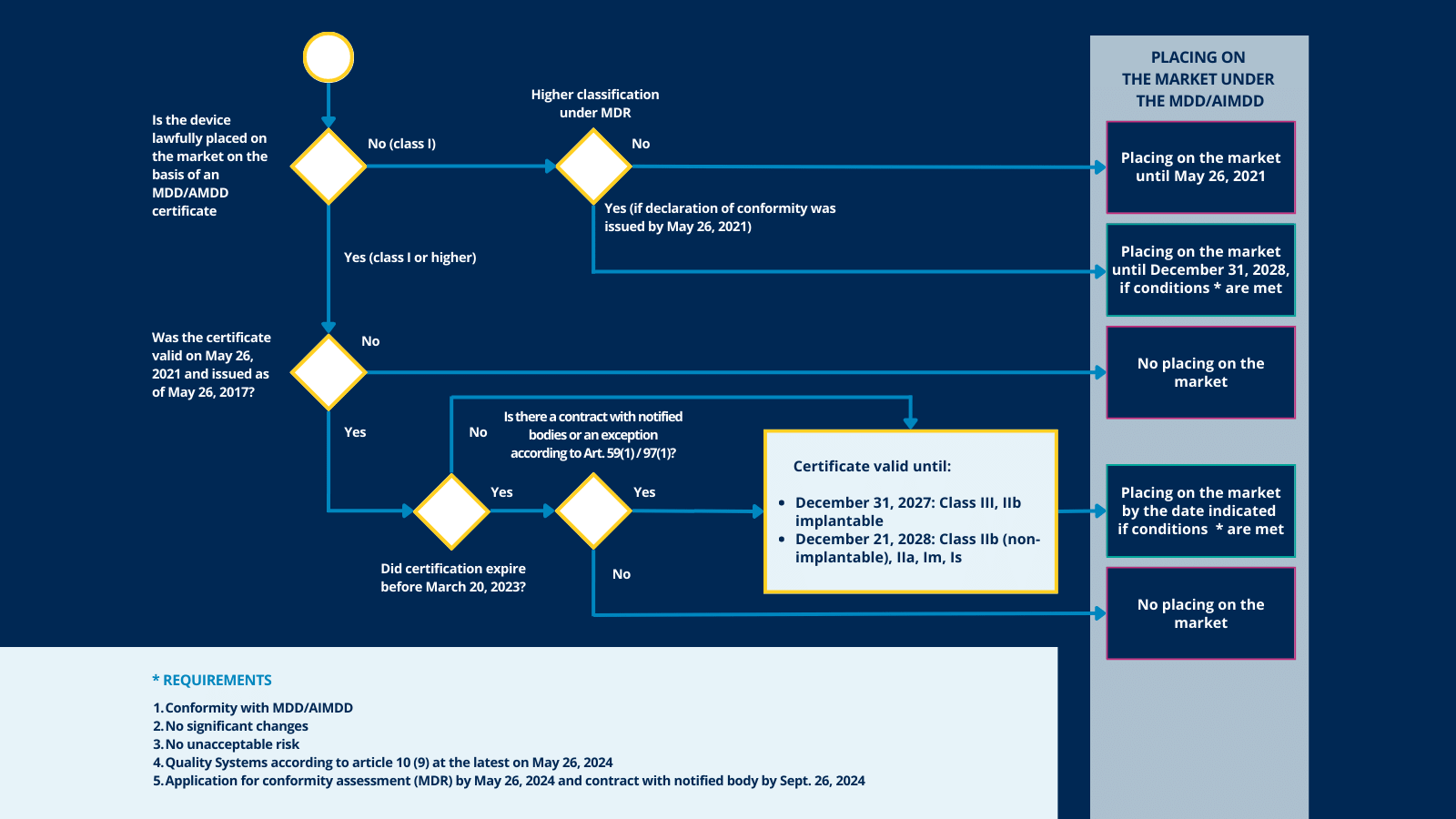
![ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services](https://medidee.com/wp-content/uploads/2022/08/Technical-Documentation-Infographic.png)
ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services

CataloniaBio & HealthTech | Hard Reg Café "Open talk with EU Notified Bodies about the extra transition year to implement MDR" (Regulatory Affairs Workgroup)

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

EU Finalizes New Medical Device Regulations (MDR) which update the regulatory framework for the marketing of devices and IVDs in Europe – Catchtrial
![Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2021/04/ELM-320-01-Requirements-Relating-to-Notified-Bodies-for-EU-MDR.png)
Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses